SELECTED IMPORTANT SAFETY INFORMATION: KOVALTRY®
is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, to any of the excipients, or to mouse or hamster proteins.
Before reviewing the cross-over study examining the PK characteristics of KOVALTRY® and Advate®,
it will be helpful to understand the PK parameters of KOVALTRY® from the LEOPOLD I trial.
PK parameters of KOVALTRY® in the LEOPOLD I trial (arithmetic mean ± SD)1
PK parameters were measured using chromogenic substrate assay after a single 50 IU/kg dose of KOVALTRY® in 21 previously treated patients ≥18 years old
AUC=area under the curve
CL=clearance
Cmax=maximum concentration
LEOPOLD=Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease
MRTIV=mean residence time after intravenous administration
PK=pharmacokinetics
SD=standard deviation
t1/2=half-life
Vss=apparent volume of distribution at steady state
KOVALTRY® and Advate® cross-over PK Study Design
| Study summary2,3 | |
| Study description | The PK profiles of KOVALTRY® and Advate® were compared in a single-dose, open-label, randomized, cross-over study Previously treated male patients aged 18 to 65 years (N=18) with severe hemophilia A |
| Dosing |
Patients were randomized to a single infusion of KOVALTRY® (50 IU/kg) (n=9) or Advate® (50 IU/kg) (n=9) Patients were then crossed over to a single infusion of the other treatment, with time for washout 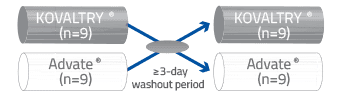
|
| PK assessment | Plasma samples were collected predose and at 0.25, 0.5, 1, 3, 6, 8, 24, 30, and 48 hours postdose for PK assessment |
PK=pharmacokinetics.
KOVALTRY® and Advate® cross-over PK study results (N=18)
Compared to Advate®, KOVALTRY® demonstrated, with statistical significance2:

higher AUC0-inf


| Cross-over PK study results (geometric mean [%CV]) following single-dose administration (50 IU/kg) of KOVALTRY® and Advate® in 18 patients2 | |||
| Chromogenic assay | KOVALTRY® | Advate® | P value |
| AUC0-last (IU•h/dL) |
2200 (23.9) |
1550 (27.4) |
<0.0001 |
| AUC0-inf (IU•h/dL) |
2440 (28.5) |
1650 (31.0) |
<0.0001 |
| Cmax (IU/dL) |
151 (19.9) |
153 (17.1) |
0.32 |
| t1/2 (h) |
13.9 (25.1) |
12.0 (23.3) |
<0.0001 |
| MRT (h) |
19.2 (27.4) |
15.0 (27.9) |
<0.0001 |
| CL (dL/h/kg) |
0.021 (28.5) |
0.030 (31.0) |
<0.0001 |
| Vss (dL/kg) |
0.39 (19.1) |
0.46 (16.7) |
<0.0001 |
|
AUC0-inf=area under the curve from time 0 to infinity. AUC0-last=area under the curve from time 0 to the last data point. |
CL=clearance. Cmax=maximum concentration. CV=coefficient of variation. MRT=mean residence time. |
PK=pharmacokinetics. t1/2=half-life. Vss=apparent volume of distribution at steady state. |
AUC0-inf=area under the curve from time 0 to infinity.
AUC0-last=area under the curve from time 0 to the last data point.
CL=clearance.
Cmax=maximum concentration.
CV=coefficient of variation.
MRT=mean residence time.
PK=pharmacokinetics.
t1/2=half-life.
Vss=apparent volume of distribution at steady state.
FVIII plasma concentration2,3
FVIII levels were 121% higher for KOVALTRY® at 48 hours compared to Advate®
FVIII levels through 48 hours (N=18)
Data are geometric means, based on chromogenic assay.
FVIII=Factor VIII.
PK=pharmacokinetics.
Median time to target FVIII threshold levels with KOVALTRY® vs Advate®2
Estimated from a population PK model (N=18)2*

PK=pharmacokinetics.
*Adapted from Shah et al. A population PK model was developed based on data obtained by a one-stage assay to simulate time to reach FVIII thresholds of 1, 3, 5 and 10% FVIII.2
Median Time to Target Threshold Levels2
Median modeled time to threshold (hours)
- 0
- 20
- 40
- 60
- 80100
Target % FVIII
- Advate®50 IU/kg (N=18)
- KOVALTRY®50 IU/kg (N=18)
A similar trend in median time to threshold was observed for 25, 30, and 40 IU/kg doses.2
PK=pharmacokinetics.
*Adapted from Shah et al. A population PK model was developed based on data obtained by a one-stage assay to simulate time to reach FVIII thresholds of 1, 3, 5 and 10% FVIII.2
INDICATION FOR KOVALTRY®
KOVALTRY® Antihemophilic Factor (Recombinant) is a recombinant human DNA sequence derived, full length Factor VIII concentrate indicated for use in adults and children with hemophilia A for:
On-demand treatment and control of bleeding episodes
Perioperative management of bleeding
Routine prophylaxis to reduce the frequency of bleeding episodes
KOVALTRY is not indicated for the treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
KOVALTRY is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, to any of the excipients, or to mouse or hamster proteins.
Hypersensitivity reactions, including anaphylaxis, are possible with KOVALTRY. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. Discontinue KOVALTRY if symptoms occur and seek immediate emergency treatment.
KOVALTRY may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Neutralizing antibody (inhibitor) formation has occurred following administration of KOVALTRY. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all Factor VIII products. Carefully monitor patients for the development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor.
Hemophilic patients with cardiovascular risk factors or diseases may be at the same risk to develop cardiovascular events as non-hemophilic patients when clotting has been normalized by treatment with Factor VIII.
Catheter-related infections may occur when KOVALTRY is administered via central venous access devices (CVADs). These infections have not been associated with the product itself.
The most frequently reported adverse reactions in clinical trials (≥5%) were inhibitors in previously untreated patients (PUPs)/minimally treated patients (MTPs), and pyrexia, headache, and rash.
For additional important risk and use information, please see full Prescribing Information.
References: 1. KOVALTRY® [prescribing information]. Whippany, NJ: Bayer HealthCare LLC; 2021. 2. Shah A, Solms A, Garmann D, et al. Improved pharmacokinetics with BAY 81-8973 versus antihemophilic factor (recombinant) plasma/albumin-free method: a randomized pharmacokinetic study in patients with severe hemophilia A [published online December 22, 2016]. Clin Pharmacokinet. doi:10.1007/s40262-016-0492-2. 3. Data on file. Bayer HealthCare Pharmaceuticals, Inc; 2016.



